- Research
- Open access
- Published:
Association between ursodeoxycholic acid use and COVID-19 in individuals with chronic liver disease: a nationwide case-control study in South Korea
Virology Journal volume 21, Article number: 202 (2024)
Abstract
Background
Conflicting evidence exists regarding the effects of ursodeoxycholic acid (UDCA) on coronavirus disease 2019 (COVID-19). This study investigates the association between UDCA administration and COVID-19 infection and its related outcomes in individuals with chronic liver disease (CLD).
Methods
A customized COVID-19 research database (n = 3,485,376) was created by integrating data from the National Health Insurance Service (NHIS) and the Korea Disease Control and Prevention Agency’s COVID-19 databases. The study focused on patients diagnosed with COVID-19 in 2021, using the NHIS data from 365 days before diagnosis. To create comparable groups with and without UDCA administration before COVID-19, we used propensity score matching. The primary endpoint was the first confirmed positive result for severe acute respiratory syndrome coronavirus-2. In addition, we identified severe COVID-19-related outcomes. Subgroup analysis were conducted based on the dose of UDCA exposure.
Results
Data from 74,074 individuals with CLD was analyzed. The participants’ average age was 57.5 years, and 52.1% (19,277) of those in each group were male. Those with prior UDCA exposure had a significantly lower risk of COVID-19 infection (adjusted OR: 0.80, 95% CI [0.76–0.85]) compared to the non-UDCA group. Additionally, the UDCA group had a lower risk of severe COVID-19 outcomes (adjusted OR: 0.67, 95% CI [0.46–0.98]). Subgroup analyses indicated that there was a decrease in COVID-19 infection and its related outcomes with increasing UDCA exposure dose.
Conclusions
Our large observational study highlights the potential use of readily available UDCA as an adjunctive therapy for COVID-19 in individuals with CLD.
Introduction
Since its declaration as a pandemic by the World Health Organization (WHO) in March 2020, COVID-19 has posed a significant challenge to public health, social stability, and the economy [1, 2]. Notably, various factors, such as chronic comorbidities, complications, and demographics, can affect the outcomes of COVID-19 [3, 4]. Specifically, individuals with chronic liver disease (CLD), particularly cirrhosis, have higher rates of morbidity and mortality from COVID-19 [5,6,7,8,9]. Vaccines and medications have been developed to reduce infection rates and prevent progression to severe disease; however, there is still a need for safer, more effective, and more accessible treatment options for individuals with CLD due to the limited duration of vaccine protection and potential side effects of medications [10,11,12,13].
Efforts were made to identify therapeutic targets through drug repurposing shortly after COVID-19 was declared a pandemic, leading to research into a prophylactic treatment approach by modulating angiotensin-converting enzyme 2 (ACE2), a critical host receptor of the virus [14]. Brevini et al. showed that ursodeoxycholic acid (UDCA), which has the farnesoid X receptor (FXR) antagonistic effects, downregulates ACE2 expression in experiments using animals and donor organs unsuitable for transplantation [15]. However, subsequent real-world retrospective studies on the relationship between UDCA intake and COVID-19 outcomes have yielded mixed results, with some studies showing positive effects [16,17,18] and others showing no significant impact [19,20,21,22].
This study explored the association between UDCA consumption and COVID-19 within a tailored South Korean COVID-19 cohort of 3,485,376 participants (including 580,896 COVID-19 cases and 2,904,480 controls). The investigation prioritized assessing the effects of UDCA consumption on COVID-19 susceptibility and its consequent outcomes among individuals with CLD within the cohort, while accounting for both the presence or absence of UDCA intake and its dosage, if applicable.
Methods
Data source and study population
A specialized COVID-19 research database was established for this investigation. This extensive repository amalgamates data from two primary origins: the National Health Insurance Service (NHIS) database, encompassing medical claims data for 97% of the Korean populace, and the database on COVID-19 confirmations and vaccinations administered by the Korea Disease Control and Prevention Agency [23]. The NHIS database furnishes a plethora of information, encompassing details regarding diagnoses, prescriptions, procedures, surgeries, insurance disbursements, and healthcare utilization for both inpatients and outpatients. It also incorporates invaluable health screening data, such as laboratory tests, physical measurements, and self-reported questionnaires concerning lifestyle habits. A tailored database was curated, incorporating data from patients diagnosed with COVID-19 between 2020 and 2021, alongside fivefold the number of controls matched for both sex and age with the diagnosed patients.
To ensure clear and efficient data analysis, our analysis only included patients diagnosed with COVID-19 between January 1, 2021, and December 31, 2021, due to the lack of definitive information on COVID-19 diagnosis dates in 2020. The date of diagnosis was defined as the index date, and only participants with NHIS data available from 365 days before the index date were included, particularly for health screening data. COVID-19-related outcomes were monitored until March 31, 2022, which customized the COVID-19 research database provided. Additionally, we utilized the International Classification of Diseases, 10th Revision (ICD-10) codes to differentiate between CLD subtypes. Finally, we matched the COVID-19 and control groups based on propensity scores. Moreover, to investigate the association between UDCA and COVID-19-related outcomes, we extracted individuals with CLD and COVID-19 and matched the event and control groups based on propensity scores.
UDCA exposure
UDCA exposure data encompassing UDCA prescription details (daily dose and duration of prescription) for the 365 days preceding the index date were retrieved. Cumulative exposure metrics, specifically cumulative defined daily dose (cDDD) and cumulative exposure duration (cED), were computed for each participant utilizing the World Health Organization’s established daily defined dose (DDD) of 750 mg/day for UDCA [24]. For analytical purposes, participants were stratified into two cohorts: those with prior UDCA exposure and those without. Moreover, participants were further segmented based on UDCA exposure duration using cDDD and cED. The study cohort was delineated into subgroups characterized by exposure durations of in less than one month (0.75 × 30 = 22.5 for cDDD, 30 days for cED), ≥ 1 month to < 3 months (0.75 × 90 = 67.5 for cDDD, 90 days for cED), and ≥ 3 months.
Outcome
The primary endpoint encompassed the initial occurrence of a positive outcome for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) utilizing reverse transcriptase–polymerase chain reaction (RT-PCR) assays conducted on nasopharyngeal or oropharyngeal swabs. Apart from the principal outcome, we explored various other complications associated with COVID-19 as secondary endpoints. These included mortality attributable to COVID-19, instances of cardiopulmonary resuscitation (M15, M587), the requirement for mechanical ventilation (M585, M5860), renal replacement therapy (O70), extracorporeal membrane oxygenation (O190), and admission to an intensive care unit for critical care (AJ).
Covariate
Demographic information, including age, sex, and income level, was extracted, with income level divided into four quartiles. Underlying diseases (hypertension, diabetes, and dyslipidemia) were assessed based on diagnoses recorded in the NHIS database up to 1 year prior to the COVID-19 diagnosis. Moreover, the Charlson comorbidity index (CCI) was utilized to gauge the burden of comorbidities [25]. CLD diagnoses were categorized as chronic viral infection, chronic liver disease, or liver cirrhosis utilizing ICD-10 codes [26]. Evaluated medications included those for hypertension and diabetes, statins, aspirin, antivirals for chronic hepatitis B, and hepatoprotective agents. Health screening results encompassed body mass index (BMI), systolic and diastolic blood pressure, fasting blood glucose, hemoglobin, glomerular filtration rate (GFR), and liver enzyme levels (aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyl transpeptidase). Additionally, participants’ current smoking status, alcohol consumption, and regular exercise habits were assessed via a self-reported questionnaire. Study participants were deemed vaccinated against COVID-19 if they had received at least one dose of any vaccine type. Supplementary Table 1 offers further details about the extracted covariates.
Statistical analysis
Baseline characteristics were expressed as mean ± standard deviation for continuous variables and as numbers with percentages (%) for categorical variables. Propensity score matching (PSM) was conducted at a 1:1 ratio, encompassing multiple covariates, such as sex, age, income level, underlying diseases, CCI, COVID-19 vaccination status, medications, BMI, systolic and diastolic blood pressure, fasting blood glucose, hemoglobin levels, GFR, liver enzyme levels, smoking status, alcohol consumption, and regular exercise habits. Exact matching was employed for sex, chronic viral infection, chronic liver disease, liver cirrhosis, COVID-19 vaccination status, antivirals for chronic hepatitis B, and hepatoprotective agents. However, greedy nearest neighbor matching was utilized for other variables, with a caliper set at 0.01 of the propensity scores. The standardized mean difference before and after PSM was utilized to assess the balance of covariate distribution between groups. Subsequently, odds ratios (ORs) and 95% confidence intervals (CIs) were computed through conditional logistic regression analysis post-matching. Additionally, multivariate-adjusted conditional logistic regression analysis was performed, incorporating the covariates. Statistical analyses were executed using SAS Enterprise Guide version 8.3 (SAS Institute Inc., Cary, NC, USA) and R 4.3.0 (R Foundation for Statistical Computing, Vienna, Austria). A significance level of P < 0.05 was considered statistically significant.
Results
Study population
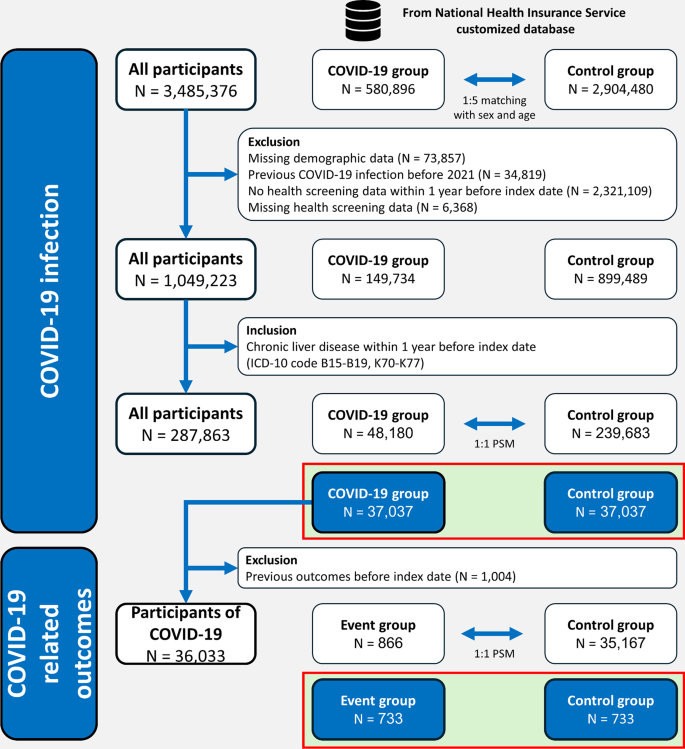
This study utilized a customized COVID-19 research database comprising 3,485,376 participants, including 580,896 confirmed COVID-19 cases and 2,904,480 control participants. After excluding individuals with missing demographic information (n = 73,857), prior COVID-19 infection before 2021 (n = 34,819), incomplete health screening data within 1 year of the index date (n = 2,321,109), or missing health screening data (n = 6,368), 1,049,223 participants remained. From among these remaining participants, individuals diagnosed with CLD using ICD-10 codes were then identified (n = 287,863).
PSM was employed to explore the association between UDCA exposure and COVID-19 infection. This technique matched participants with CLD in a 1:1 ratio to those with COVID-19 (n = 37,037) and control groups (n = 37,037) (Table 1). Subsequently, to investigate the relationship between UDCA exposure and COVID-19-related outcomes, another 1:1 PSM was performed within the previously matched group to separate participants with and without COVID-19-related outcomes (Baseline characteristics in Supplementary Table 2). The schematic diagram for this case-control study is presented in Fig. 1. Table 1 presents the comparison results of the characteristics of the COVID-19 and control groups before and after PSM.
COVID-19 infection according to UDCA exposure
Table 2 displays the OR and 95% CIs for COVID-19 infection in relation to UDCA exposure. Participants exposed to UDCA exhibited an adjusted OR of 0.80 for COVID-19 infection (95% CI [0.76–0.85], P-value < 0.001) compared with those in the non-exposure group. Upon stratification based on UDCA dose (with the cDDD < 22.5 group as the reference), the adjusted OR was 0.86 (95% CI [0.80–0.93], P-value < 0.001) for the 22.5 ≤ cDDD < 67.5 group and 0.83 (95% CI [0.77–0.90], P-value < 0.001) for the cDDD ≥ 67.5 group. Analogous outcomes were observed when analysing according to cED.
COVID-19-related outcomes according to UDCA exposure
Table 3 presents the association between UDCA exposure and COVID-19-related outcomes. Participants with UDCA exposure had an adjusted OR of 0.67 for COVID-19-related outcomes (95% CI [0.46–0.98], P-value: 0.04) compared with the non-exposure group. Following an analysis based on UDCA dose (using the cDDD < 22.5 group as reference), the adjusted OR was 0.89 (95% CI [0.50–1.58], P-value: 0.68) for the 22.5 ≤ cDDD < 67.5 group and 0.48 (95% CI [0.27–0.88], P-value: 0.02) for the cDDD ≥ 67.5 group. Similar results were found when an analysis based on cED was done. A forest plot analysis was employed to illustrate the findings presented in Tables 2 and 3 (Supplementary Fig. 1).
Sensitivity analysis
This study examined data from 365 days before the COVID-19 diagnosis, and the sensitivity analysis used data from 180 days before the COVID-19 diagnosis (Supplementary Tables 3,4). The sensitivity analysis was consistent with the main results.
Discussion
A nationwide population-based cohort study utilizing a tailored COVID-19 research database encompassing 3.4 million individuals was employed to ascertain COVID-19 infection and its associated outcomes concerning UDCA exposure and dosage after PSM following the identification of individuals with CLD. Findings revealed a favorable correlation between COVID-19 infection and its related outcomes in the exposed group compared with the unexposed group (reference) (COVID-19 infection, adjusted OR: 0.80, 95% CI [0.76–0.85]; COVID-19-related outcomes, adjusted OR: 0.67, 95% CI [0.46–0.98]). To our knowledge, this study represents the most comprehensive investigation to date into the association between UDCA and COVID-19.
Prevention of COVID-19 is crucial for individuals with CLD. These individuals face an increased risk of severe complications from COVID-19, with those having cirrhosis experiencing particularly poor outcomes [5,6,7,8,9]. This is supported by findings from the National COVID Cohort Collaborative Study and the Veterans Affairs healthcare system, both of which independently reported that COVID-19 infection raises the risk of death within 30 days by 2.38 and 1.7 times, respectively, in individuals with cirrhosis compared to those without [6, 9]. To further explore this association, we conducted a comparative analysis of COVID-19 infection and its outcomes between individuals with CLD and those with cirrhosis across our entire cohort, utilizing our customized COVID-19 research database. Our results align with previous studies [COVID-19 infection: without liver disease (reference); CLD, 1.11 (p < 0.001); cirrhosis, 1.01 (p = 0.88). COVID-19 related outcomes: without liver disease (reference); CLD, 1.79 (p < 0.001); cirrhosis, 2.75 (p < 0.001)] (Supplementary Table 5) [6, 9, 27]. Impairments in the complement system, macrophage activation, lymphocyte and neutrophil function, upregulated Toll-like receptors, and intestinal dysbiosis contribute to the increased susceptibility of individuals with CLD to viral infections. These factors trigger cytotoxic T-cell activation and dysregulation of the innate immune response, ultimately leading to liver damage and increased mortality [5, 7, 8].
UDCA is a well-established first-line treatment for primary biliary cholangitis [28,29,30]. It stimulates bile acid secretion and has shown immunomodulatory and anti-inflammatory effects in experimental studies. It also reduces oxidative stress and protects liver cells from apoptosis [31,32,33,34,35,36]. Recent research has identified a potential role for bile acids like UDCA in regulating COVID-19 infection, with a focus on the ACE2 receptor, which is a critical entry point for SARS-CoV-2 [37, 38]. Experimental studies suggest that bile acids can act on this pathway in multiple ways: (1) hindering viral entry by disrupting the interaction between ACE2 and the spike protein, (2) influencing ACE2 activity, and potentially (3) regulating ACE2 expression [15, 39, 40]. Additionally, bile acids have shown promise in modulating the cytokine storm, an essential factor in the development of acute respiratory distress syndrome (ARDS), a severe complication of COVID-19 [41, 42]. UDCA has a favorable safety profile and few side effects, making it a potential treatment to prevent infection and mitigate disease progression in patients with COVID-19 [43].
In a landmark decision on May 5, 2023, the WHO declared COVID-19 was no longer a global public health emergency, marking a turning point after a grueling 3-year battle [44,45,46]. This shift signifies that COVID-19 will transition from a pandemic to an endemic, managed alongside other prevalent illnesses. Factors contributing to this decision include rising herd immunity due to vaccination and natural infection, a reduced burden on healthcare systems, and decreased overall disease severity [44,45,46]. However, the WHO’s declaration does not signal the complete eradication of COVID-19. The emergence of new variants and the potential decline in vaccination rates pose significant challenges to ongoing management efforts [47, 48]. Therefore, continued vigilance is essential. This is especially crucial for patients with pre-existing medical conditions that may make them more vulnerable to COVID-19 or for those living in low-income countries with low vaccination rates [49]. In such instances, UDCA can be used as an additional treatment to vaccines and conventional medications, and it has been proven to be affordable and accessible [50, 51].
Limitations and strengths
This study has some limitations. First, the population’s demographic composition is predominantly from a single ethnic group. Second, the study cohort consisted mainly of individuals with CLD because UDCA was prescribed primarily to this group in South Korea. Therefore, it is difficult to explain the relationship between UDCA and COVID-19 in non-CLD groups, and the baseline characteristics of the study population differ from those of the general population. Third, identifying individuals with CLD relied solely on ICD-10 codes, which may not be perfectly accurate. Additionally, the available medical records only covered approximately 2 years. Fourth, there may be discrepancies between UCDA’s prescribed and actual usage. Patients with higher prescription rates and frequent hospital visits might focus more on preventing COVID-19, potentially influencing result interpretations [52, 53]. Fifth, although various factors were adjusted for, misclassification and residual confounding factors may still be present. Sixth, the study did not obtain results regarding SARS-CoV-2 variants or reinfections. However, despite these limitations, we found a positive association between UDCA intake and COVID-19 infection and its related outcomes among 74,074 individuals with CLD who underwent PSM. Specifically, the analysis, stratified by the level of UDCA intake using data from the year before COVID-19 infection, revealed that higher UDCA intake, rather than simply its presence or absence, was associated with more beneficial effects. Unlike COVID-19 vaccines and medications, UDCA does not need to be re-studied for adverse effects, and its relatively low cost and accessibility make it feasible even in developing countries.
In reporting this study, we do not prioritize supplementing research findings with randomized controlled trials (RCTs), as is often suggested to complement observational studies. Conducting RCTs to investigate the association between UDCA and COVID-19 in the current situation, unlike during past severe pandemics, is unrealistic and of little significance. However, we aim to provide helpful information for patients with limited access to COVID-19 vaccines and medications by reporting positive outcomes of UDCA intake in patients with CLD using a large observational study. In addition, we hope that this study will contribute to the discussion of UDCA administration in situations with viruses similar to SARS-CoV-2 in the future [54].
Conclusions
This large-scale observational study has shown that UDCA can reduce COVID-19 infection and its related outcomes in individuals with CLD. These findings suggest that the readily available UDCA could be a valuable addition to the treatment regimens of individuals with CLD susceptible to COVID-19.
Data availability
No datasets were generated or analysed during the current study.
References
Bedford J, Enria D, Giesecke J, Heymann DL, Ihekweazu C, Kobinger G, Lane HC, Memish Z, Oh MD, Sall AA, et al. COVID-19: towards controlling of a pandemic. Lancet. 2020;395:1015–8.
Naseer S, Khalid S, Parveen S, Abbass K, Song H, Achim MV. COVID-19 outbreak: impact on global economy. Front Public Health. 2022;10:1009393.
Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021;21:855.
Choi YJ, Park JY, Lee HS, Suh J, Song JY, Byun MK, Cho JH, Kim HJ, Park HJ. Variable effects of underlying diseases on the prognosis of patients with COVID-19. PLoS ONE. 2021;16:e0254258.
Baldelli L, Marjot T, Barnes E, Barritt AS, Webb GJ, Moon AM. SARS-CoV-2 infection and liver disease: a review of Pathogenesis and outcomes. Gut Liver. 2023;17:12–23.
Ioannou GN, Liang PS, Locke E, Green P, Berry K, O’Hare AM, Shah JA, Crothers K, Eastment MC, Fan VS, Dominitz JA. Cirrhosis and severe Acute Respiratory Syndrome Coronavirus 2 infection in US veterans: risk of infection, hospitalization, Ventilation, and Mortality. Hepatology. 2021;74:322–35.
Spearman CW, Aghemo A, Valenti L, Sonderup MW. COVID-19 and the liver: a 2021 update. Liver Int. 2021;41:1988–98.
Marjot T, Webb GJ, Barritt ASt, Moon AM, Stamataki Z, Wong VW, Barnes E. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18:348–64.
Ge J, Pletcher MJ, Lai JC. Outcomes of SARS-CoV-2 infection in patients with chronic liver disease and cirrhosis: a National COVID Cohort Collaborative Study. Gastroenterology. 2021;161:1487–e15011485.
Moon AM, Webb GJ, García-Juárez I, Kulkarni AV, Adali G, Wong DK, Lusina B, Dalekos GN, Masson S, Shore BM, et al. SARS-CoV-2 infections among patients with Liver Disease and Liver Transplantation who received COVID-19 vaccination. Hepatol Commun. 2022;6:889–97.
Cromer D, Steain M, Reynaldi A, Schlub TE, Khan SR, Sasson SC, Kent SJ, Khoury DS, Davenport MP. Predicting vaccine effectiveness against severe COVID-19 over time and against variants: a meta-analysis. Nat Commun. 2023;14:1633.
Zampino R, Mele F, Florio LL, Bertolino L, Andini R, Galdo M, De Rosa R, Corcione A, Durante-Mangoni E. Liver injury in remdesivir-treated COVID-19 patients. Hepatol Int. 2020;14:881–3.
Wong GL, Hui VW, Yip TC, Lui GC, Hui DS, Wong VW. Minimal risk of Drug-Induced Liver Injury with Molnupiravir and Ritonavir-Boosted Nirmatrelvir. Gastroenterology. 2023;164:151–3.
Gaziano L, Giambartolomei C, Pereira AC, Gaulton A, Posner DC, Swanson SA, Ho Y-L, Iyengar SK, Kosik NM, Vujkovic M, et al. Actionable druggable genome-wide mendelian randomization identifies repurposing opportunities for COVID-19. Nat Med. 2021;27:668–76.
Brevini T, Maes M, Webb GJ, John BV, Fuchs CD, Buescher G, Wang L, Griffiths C, Brown ML, Scott WE, et al. FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2. Nature. 2023;615:134–42.
Li Y, Zhu N, Cui X, Lin Y, Li X. Protective effect of ursodeoxycholic acid on COVID-19 in patients with chronic liver disease. Front Cell Infect Microbiol. 2023;13:1178590.
John BV, Bastaich D, Webb G, Brevini T, Moon A, Ferreira RD, Chin AM, Kaplan DE, Taddei TH, Serper M, et al. Ursodeoxycholic acid is associated with a reduction in SARS-CoV-2 infection and reduced severity of COVID-19 in patients with cirrhosis. J Intern Med. 2023;293:636–47.
Hu L, Zhang H, Huang C, Shen T, Feng Z, Mu F, Xu L, Lin Y, Yue C, Guo K, et al. Effect of Ursodeoxycholic Acid on preventing SARS-CoV-2 infection in patients with liver transplantation: a multicenter retrospective cohort study. Qjm; 2023.
Colapietro F, Angelotti G, Masetti C, Shiffer D, Pugliese N, De Nicola S, Carella F, Desai A, Ormas M, Calatroni M et al. Ursodeoxycholic Acid Does Not Improve COVID-19 Outcome in Hospitalized Patients. Viruses : 2023, 15.
Liu T, Wang JS. Ursodeoxycholic acid administration did not reduce susceptibility to SARS-CoV-2 infection in children. Liver Int. 2023;43:1950–4.
Marrone G, Covino M, Merra G, Piccioni A, Amodeo A, Novelli A, Murri R, Pompili M, Gasbarrini A, Franceschi F. Ursodeoxycholic acid does not affect the clinical outcome of SARS-CoV-2 infection: a retrospective study of propensity score-matched cohorts. Liver Int. 2024;44:83–92.
Corpechot C, Verdoux M, Frank-Soltysiak M, Duclos-Vallée JC, Grimaldi L. Exploring the impact of ursodeoxycholic acid therapy on COVID-19 in a real-word setting. J Med Virol. 2024;96:e29418.
Nation Health Insurance Data Sharing Service. 2024. [https://nhiss.nhis.or.kr/bd/ab/bdaba000eng.do]
ATC/DDD, Index. 2024. [https://atcddd.fhi.no/atc_ddd_index/code=A05AA02]
Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel JM, Sundararajan V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–82.
Lee SR, Lee HJ, Choi EK, Han KD, Jung JH, Cha MJ, Oh S, Lip GYH. Direct oral anticoagulants in patients with Atrial Fibrillation and Liver Disease. J Am Coll Cardiol. 2019;73:3295–308.
Fan VS, Dominitz JA, Eastment MC, Locke ER, Green P, Berry K, O’Hare AM, Shah JA, Crothers K, Ioannou GN. Risk factors for testing positive for severe Acute Respiratory Syndrome Coronavirus 2 in a National United States Healthcare System. Clin Infect Dis. 2021;73:e3085–94.
Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary biliary cholangitis: 2018 Practice Guidance from the American Association for the study of Liver diseases. Hepatology. 2019;69:394–419.
Lee HA, Chang Y, Sung PS, Yoon EL, Lee HW, Yoo J-J, Lee Y-S, An J, Song DS, Cho YY, et al. Therapeutic mechanisms and beneficial effects of non-antidiabetic drugs in chronic liver diseases. Clin Mol Hepatol. 2022;28:425–72.
Chang J-I, Kim JH, Sinn DH, Cho J-Y, Kim KM, Oh JH, Park Y, Sohn W, Goh MJ, Kang W, et al. Clinical outcomes and validation of Ursodeoxycholic Acid Response scores in patients with Korean primary biliary cholangitis: a Multicenter Cohort Study. Gut Liver. 2023;17:620–8.
Poupon R. Ursodeoxycholic acid and bile-acid mimetics as therapeutic agents for cholestatic liver diseases: an overview of their mechanisms of action. Clin Res Hepatol Gastroenterol. 2012;36(Suppl 1):S3–12.
Oh AR, Bae JS, Lee J, Shin E, Oh BC, Park SC, Cha JY. Ursodeoxycholic acid decreases age-related adiposity and inflammation in mice. BMB Rep. 2016;49:105–10.
Takigawa T, Miyazaki H, Kinoshita M, Kawarabayashi N, Nishiyama K, Hatsuse K, Ono S, Saitoh D, Seki S, Yamamoto J. Glucocorticoid receptor-dependent immunomodulatory effect of ursodeoxycholic acid on liver lymphocytes in mice. Am J Physiol Gastrointest Liver Physiol. 2013;305:G427–438.
Okada K, Shoda J, Taguchi K, Maher JM, Ishizaki K, Inoue Y, Ohtsuki M, Goto N, Takeda K, Utsunomiya H, et al. Ursodeoxycholic acid stimulates Nrf2-mediated hepatocellular transport, detoxification, and antioxidative stress systems in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G735–747.
El-Sherbiny GA, Taye A, Abdel-Raheem IT. Role of ursodeoxycholic acid in prevention of hepatotoxicity caused by Amoxicillin-clavulanic acid in rats. Ann Hepatol. 2009;8:134–40.
Mueller M, Castro RE, Thorell A, Marschall HU, Auer N, Herac M, Rodrigues CMP, Trauner M. Ursodeoxycholic acid: effects on hepatic unfolded protein response, apoptosis and oxidative stress in morbidly obese patients. Liver Int. 2018;38:523–31.
Beyerstedt S, Casaro EB, Rangel ÉB. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis. 2021;40:905–19.
Fiorucci S, Urbani G, Biagioli M, Sepe V, Distrutti E, Zampella A. Bile acids and bile acid activated receptors in the treatment of Covid-19. Biochem Pharmacol 2023:115983.
Carino A, Moraca F, Fiorillo B, Marchianò S, Sepe V, Biagioli M, Finamore C, Bozza S, Francisci D, Distrutti E, et al. Hijacking SARS-CoV-2/ACE2 receptor Interaction by Natural and semi-synthetic Steroidal agents acting on functional pockets on the receptor binding domain. Front Chem. 2020;8:572885.
Fiorillo B, Marchianò S, Moraca F, Sepe V, Carino A, Rapacciuolo P, Biagioli M, Limongelli V, Zampella A, Catalanotti B, Fiorucci S. Discovery of bile acid derivatives as potent ACE2 activators by virtual screening and essential dynamics. J Chem Inf Model. 2022;62:196–209.
Ko WK, Lee SH, Kim SJ, Jo MJ, Kumar H, Han IB, Sohn S. Anti-inflammatory effects of ursodeoxycholic acid by lipopolysaccharide-stimulated inflammatory responses in RAW 264.7 macrophages. PLoS ONE. 2017;12:e0180673.
Abdulrab S, Al-Maweri S, Halboub E. Ursodeoxycholic acid as a candidate therapeutic to alleviate and/or prevent COVID-19-associated cytokine storm. Med Hypotheses. 2020;143:109897.
Prayitno K, Bhat M. Repurposing UDCA, an FXR inhibitor, to prevent SARS-Cov-2 infection. Gastroenterology. 2023;164:1019–20.
Contreras S, Iftekhar EN, Priesemann V. From emergency response to long-term management: the many faces of the endemic state of COVID-19. Lancet Reg Health Eur. 2023;30:100664.
Burki T. WHO ends the COVID-19 public health emergency. Lancet Respir Med. 2023;11:588.
Sarker R, Roknuzzaman ASM, Hossain MJ, Bhuiyan MA, Islam MR. The WHO declares COVID-19 is no longer a public health emergency of international concern: benefits, challenges, and necessary precautions to come back to normal life. Int J Surg. 2023;109:2851–2.
Hadj Hassine I. Covid-19 vaccines and variants of concern: a review. Rev Med Virol. 2022;32:e2313.
Sohan M, Hossain MJ, Islam MR. The SARS-CoV-2 Omicron (B.1.1.529) variant and effectiveness of existing vaccines: what we know so far. J Med Virol. 2022;94:1796–8.
Barouch DH. Covid-19 vaccines - immunity, variants, boosters. N Engl J Med. 2022;387:1011–20.
Omer SB, Salmon DA, Orenstein WA, deHart MP, Halsey N. Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. N Engl J Med. 2009;360:1981–8.
Roy DN, Biswas M, Islam E, Azam MS. Potential factors influencing COVID-19 vaccine acceptance and hesitancy: a systematic review. PLoS ONE. 2022;17:e0265496.
Park HJ, Byun MK, Kim HJ, Ahn CM, Rhee CK, Kim K, Kim BY, Bae HW, Yoo KH. Regular follow-up visits reduce the risk for asthma exacerbation requiring admission in Korean adults with asthma. Allergy Asthma Clin Immunol. 2018;14:29.
Park HJ, Byun MK, Kim T, Rhee CK, Kim K, Kim BY, Ahn SI, Jo YU, Yoo KH. Frequent outpatient visits prevent exacerbation of Chronic Obstructive Pulmonary Disease. Sci Rep. 2020;10:6049.
Peeri NC, Shrestha N, Rahman MS, Zaki R, Tan Z, Bibi S, Baghbanzadeh M, Aghamohammadi N, Zhang W, Haque U. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int J Epidemiol. 2020;49:717–26.
Acknowledgements
Not applicable.
Funding
This research was supported by Dong-A University Research Fund.
Author information
Authors and Affiliations
Contributions
Sang Yi Moon and Minkook Son contributed equally to this work as first authors. Dr. S. Moon, M. Son, and Y. Baek had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: S. Moon, M. SonAcquisition, analysis, or interpretation of data: S. Moon, M. Son. Drafting of the manuscript: S. Moon, M. Son. Critical review of the manuscript for important intellectual content: Y, Kang, Y. Baek. Statistical analysis: M. Son. Administrative, technical, or material support: Y. Baek. Supervision: Y. Baek. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Dong-A University College of Medicine Institutional Review Board exempted this retrospective study from review due to its design (utilizing de-identified, publicly available clinical data for analysis) (DAUHIRB-EXP-23-026).
Consent for publication
Not applicable.
Additional information
This study used the database of the KDCA and the NHIS for policy and academic research. The research number of this study is KDCA-NHIS-2023-1-567. The KDCA is the Korea Disease Control and Prevention Agency, Republic of Korea. The NHIS is the National Health Insurance Service, Republic of Korea.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Moon, S.Y., Son, M., Kang, Y.W. et al. Association between ursodeoxycholic acid use and COVID-19 in individuals with chronic liver disease: a nationwide case-control study in South Korea. Virol J 21, 202 (2024). https://doi.org/10.1186/s12985-024-02464-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-024-02464-1